Unifying Approach
Across Our Four Pillars
We build biomarker-driven, safety -constrained closed-loop neuromodulation by integrating multimodal sensing, computational modeling, control policy design, and IRB-based translational studies.
We focus on a range of neurological conditions, particularly those that are high-impact and underserved. While our projects span biomarker discovery, computational modeling, smart clinical trials, and closed-loop control, we are currently investigating:
- Epilepsy
A chronic brain disorder characterized by recurrent seizures caused by sudden bursts of abnormal electrical activity. Seizures can affect safety, cognition, and quality of life, and a substantial subset of patients remain drug-resistant. Epilepsy affects ~50 million people worldwide.
- Chronic Pain
Pain that persists for months (typically >3 months) and can arise from nerve injury, inflammation, or altered central pain processing. It is a leading cause of disability and can substantially restrict daily activities and work. In 2021, ~51.6 million U.S. adults experienced chronic pain.
- Cognitive Disorders
We study conditions that impair attention and vigilance (sustaining focus), memory, and cognitive control, affecting learning, daily independence, and workplace performance.
AI/ML
For Biomarker Discovery
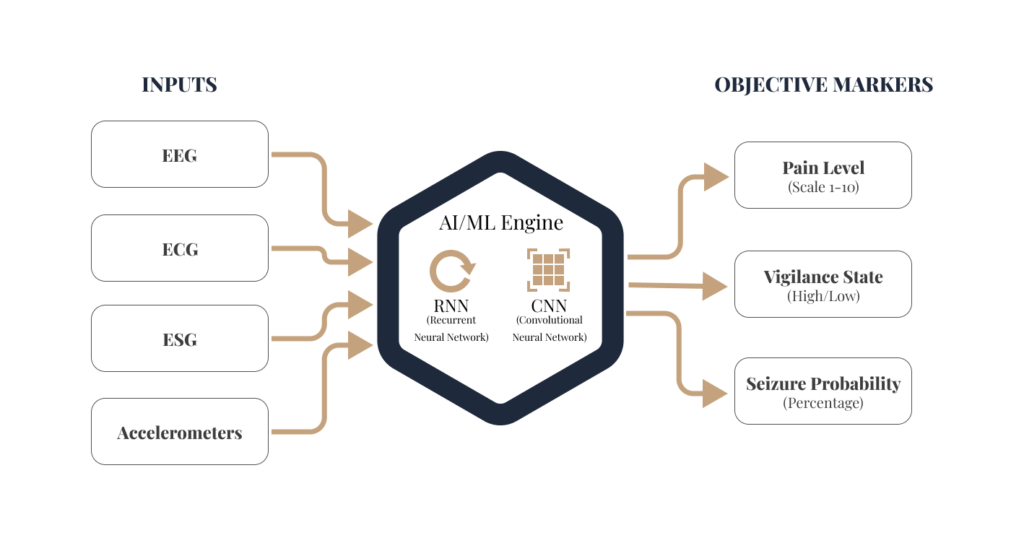
Traditional diagnostics often rely on intermittent snapshots of a patient’s state. Our approach utilizes continuous, multi-modal sensing to create a comprehensive profile of a patient’s health.
Neural Signals
We analyze high-resolution data from EEG, Electrospinogram (ESG), Evoked Potentials (EPs), and Local Field Potentials (LFPs) to assess real-time network reactivity.
Physiological & Behavioral Data
Our algorithms integrate cardiovascular and autonomic signals to identify physiological correlates of disease states.
Sensor Fusion
Using AI, we fuse these disparate data streams—ranging from central nervous system (CNS) signals to peripheral wearables—to quantify behavioral function and its impairment by underlying conditions.
Computational Modeling:
A Bridge to Precision Medicine
Our lab uses mathematical modeling to unify neural physiology, device physics, and clinical outcomes. As a newly launched lab, we build on our PI’s decades of prior research and translational development in closed-loop neuromodulation. We develop mechanistic and data-driven computational models of stimulation delivery and brain/spinal network response to predict treatment effects and optimize patient-specific neuromodulation for safety and efficacy.
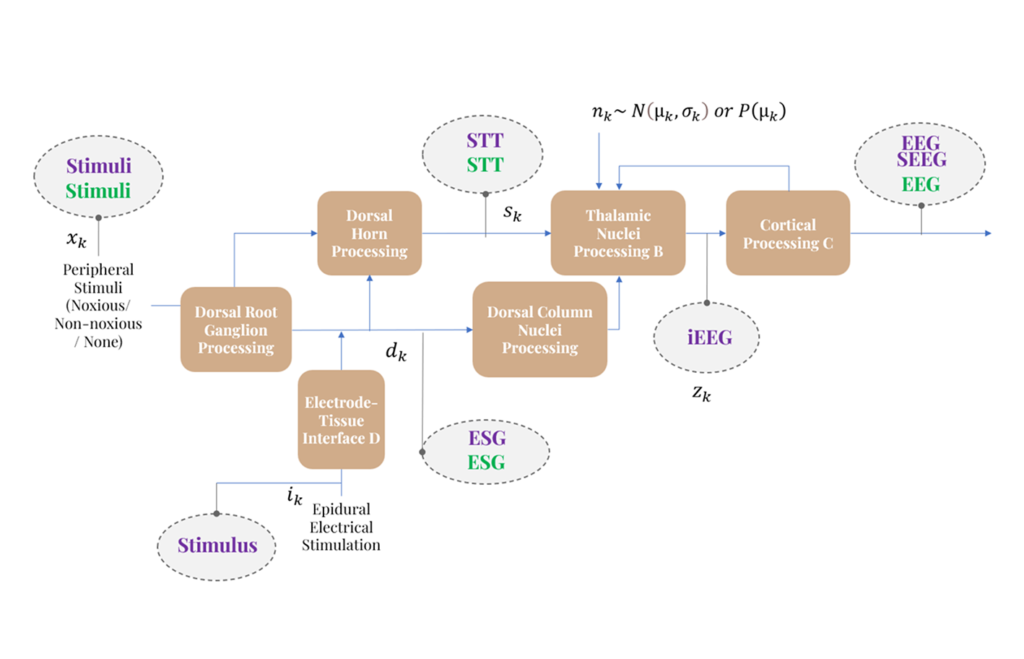
Modeling Neural Pathway Dynamics
We develop models to track how information propagates through the body to the brain.
- Pathway Mapping
- We characterize the flow of signals from a stimulus (e.g., touch or pain) to the cortex.
- Predicting Internal State
- Using system identification and state estimation, our models estimate internal dynamics and latent state variables that govern responses across inputs and conditions.
Smart Model-Predicted Personalized Dosing
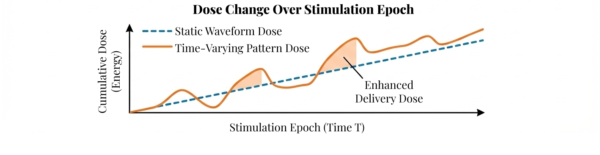
Traditional neuromodulation often relies on "static" waveforms (fixed amplitude, frequency, pulse width). We are developing time-varying pulse-pattern stimulation that adapts therapy “dose” over time.
- Dynamic Dose
- Our models adjust stimulation parameters to shape the delivered “dose rate” across time, matching the patient’s changing needs.
- Safety-constrained dosing
- We track dose metrics to keep therapy effective while staying within predefined safe limits and minimizing side effects.
Different Model Applications
We cast neuromodulation in a systems-and-control framework, where sensing and stimulation form a closed feedback loop across the brain and spinal cord.
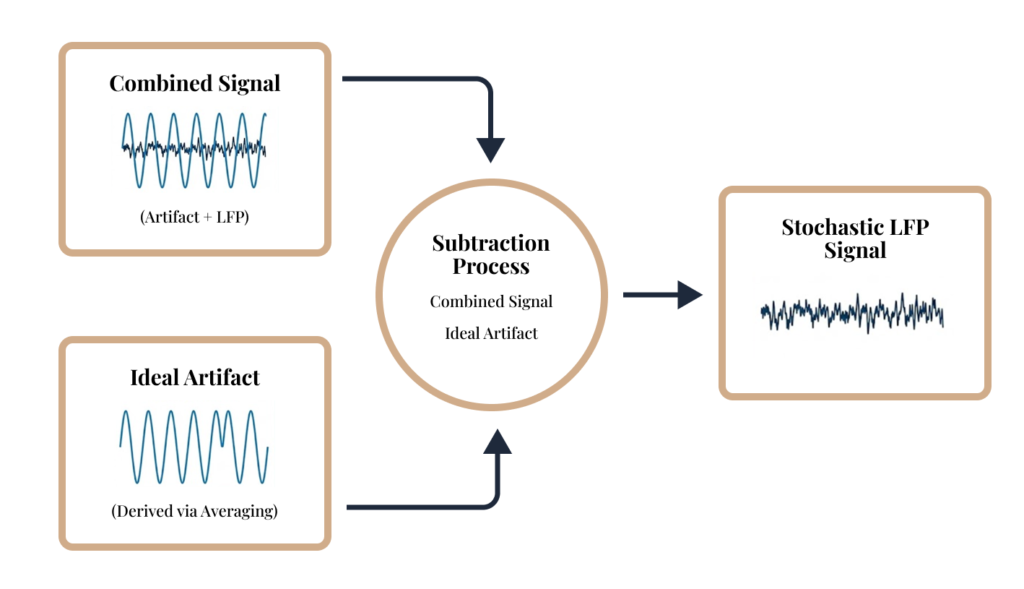
- Artifact-Robust Sensing
- We model and suppress stimulation artifacts to recover interpretable neurophysiological signals in real-time.
- Patient-Specific Models
- We translate individual neural dynamics into control-relevant features and therapy targets.
- Model-Guided Adaptation
- Using state estimation and biomarker-driven decision logic, therapy is adjusted to suppress pathological rhythms and restore network balance. Recent work we presented at AES (Dec 2025) identified seizure-termination network markers (e.g., PLV) that may reflect therapy-driven plasticity and could inform adaptive control policies.
Closed-Loop Control
Multi-Layer Control Architecture
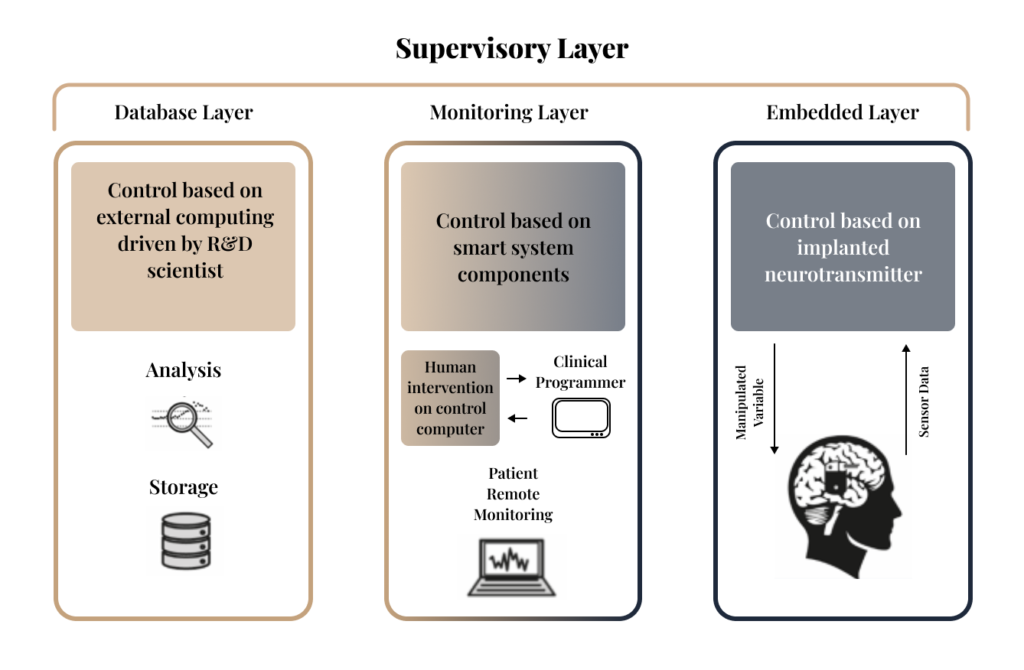
A closed-loop system is an intelligent feedback loop designed to monitor a patient's biological state and adjust therapy automatically. Our architecture organizes this process into three integrated layers:
- Embedded Layer
- This is the patient-side interface where an implanted neurostimulator records sensor data and adjusts manipulated variables to provide real-time therapy.
- Monitoring Layer
- This layer allows for human intervention, enabling clinicians to re-program the system and patients to be monitored remotely via smart components.
- Database Layer
- This is our research hub where R&D scientists perform deep analysis and manage storage to refine and improve control algorithms.
Closed Loop with Dynamic Pulse Patterns
For decades, most clinical neuromodulation has relied on time-invariant stimulation parameters.
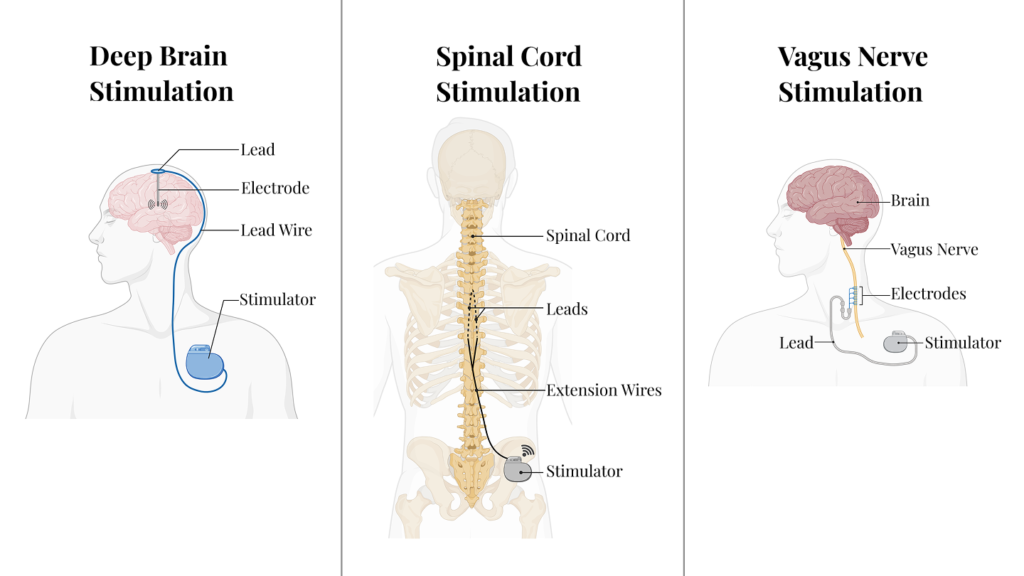
While traditional Deep Brain Stimulation (DBS), Spinal Cord Stimulation (SCS), and Vagus Nerve Stimulation (VNS) have historically relied on static pulses, our lab is advancing Dynamic Stimulation to improve patient outcomes. Our PI started these efforts with the Dynamo Study, and we are actively expanding this line of work to other neurological conditions beyond chronic pain.
Encoding Time-Varying Stimulation Patterns Across Time and/or Space
We modulate neural activation with different pulse modulations.
Changing neural activation with pulse amplitude modulation in Swine
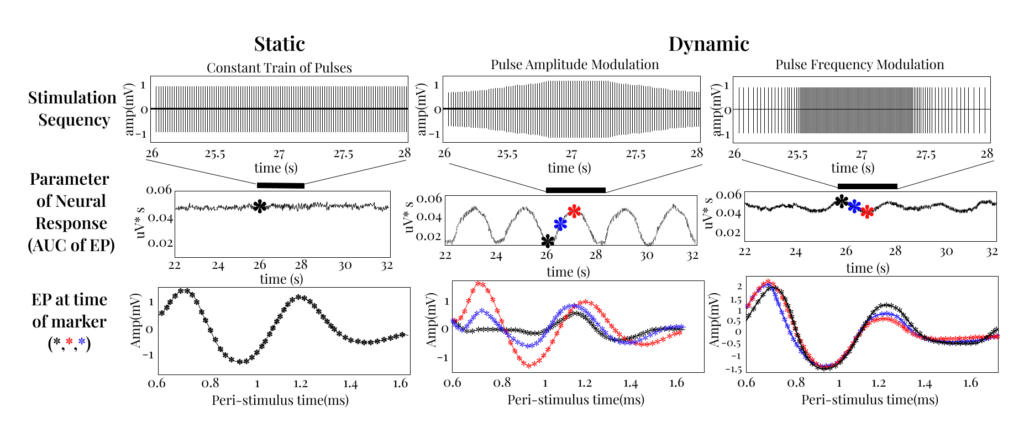
Building on our PI’s prior preclinical experience (including large-animal studies), we highlight a clear limitation of purely static stimulation and the opportunity enabled by time-varying stimulation patterns:
-
- By using dynamic pulses we can modulate the neural response over time. This allows us to activate specific neural elements in a way that is more targeted and physiologically relevant than standard static pulses.
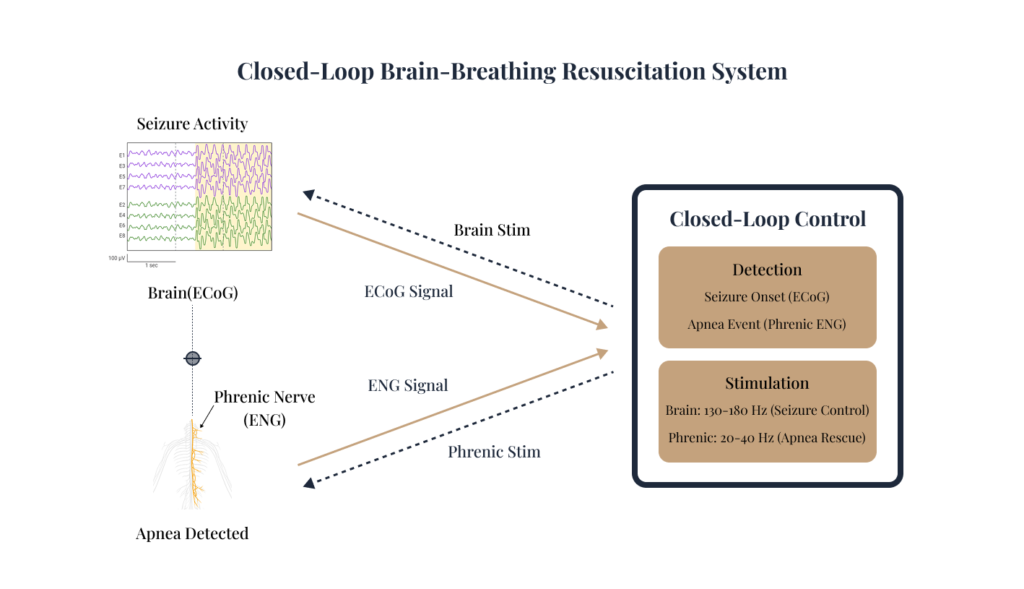
Closed-Loop Neuro-Respiratory Safety Concept
We have a collaboration with Dr. Sandipan Pati for a Closed-Loop Resuscitation System. This is a research concept that explores integrating real-time neural and respiratory sensing aiming at seizure control and apnea rescue:
- Seizure Control
- Exploring stimulation strategies intended to modulate abnormal neural activity.
- Apnea Rescue
- Targeted stimulation intended to restore breathing when natural respiration fails
This early-stage concept is focused on feasibility, safety constraints, and algorithm design for coordinated neuro-respiratory intervention.
Smart Clinical Trials
Redefining Research
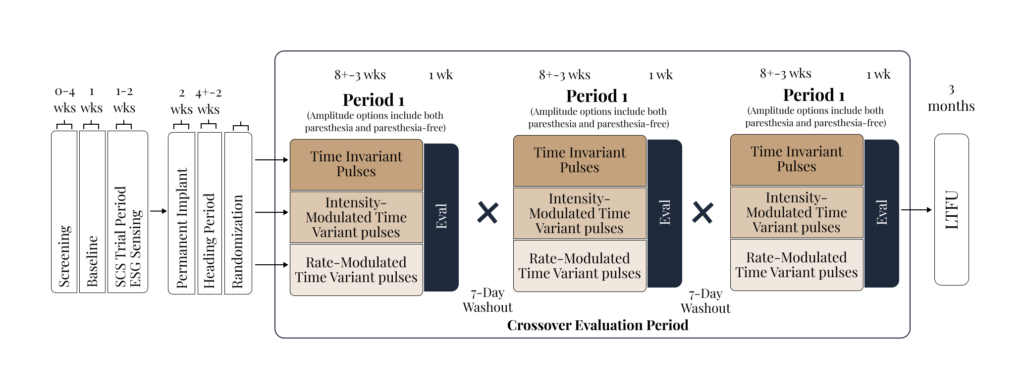
We run IRB-based study protocols designed to maximize information per participant and accelerate iteration of neuromodulation parameters and biomarkers. “Smart/Optimized” refers to: (i) standardized clinical endpoints with digital capture (e.g., ePRO/eDiary) alongside objective measures, and (iii) analysis pipelines that support rapid phenotype/state characterization and patient-specific dose–response mapping.
A Multimodal, Data-driven Approach
We are initiating in-clinic studies that pair controlled stimulation with synchronized neural + physiological recordings to quantify acute response, identify candidate biomarkers, and inform subsequent personalization and closed-loop design across neurological conditions.
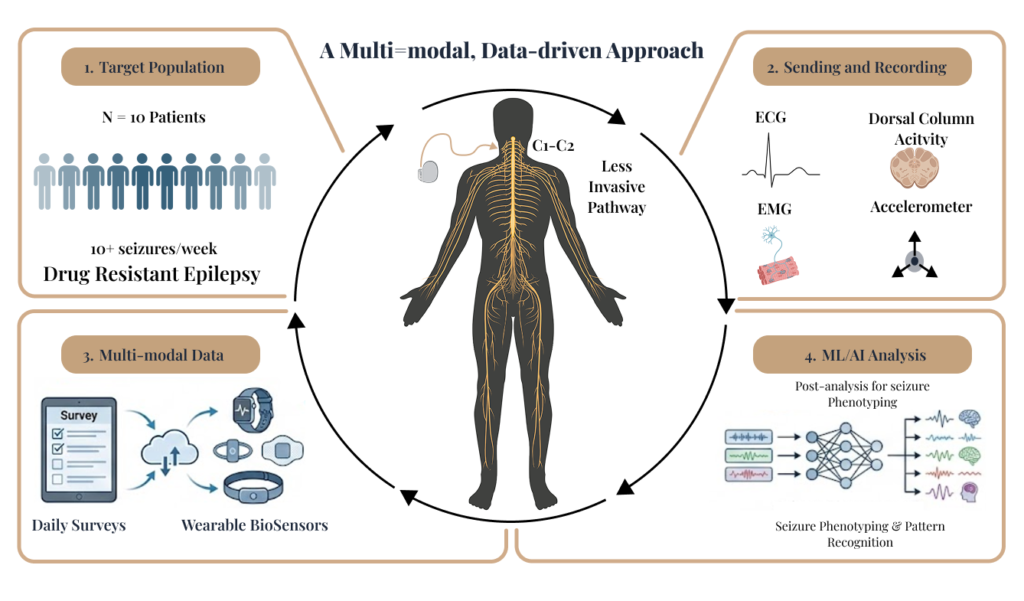
Targeting Less Invasive Pathways
Our research seeks to provide relief with less risk. We are initiating feasibility studies to evaluate novel, less invasive neural pathways for neuromodulation therapy.
Our primary research goals include:
- Safety and Efficacy
- Assessing the safety, optimal dosage, and tolerability of different neuromodulation paradigms.
- Physiological State Characterization
- Quantifying how stimulation modulates measurable neurophysiological and autonomic state markers to distinguish clinically relevant states.
- Personalized Parameters
- Characterizing individual response profiles across a broad parameter space to identify patient-specific settings that perform best.
Innovative Dynamic Stimulation Studies